When we last checked in with the Klapötke lab at Munich, it was to highlight their accomplishments in the field of nitrotetrazole oxides. Never forget, the biggest accomplishment in such work is not blowing out the lab windows. We’re talking high-nitrogen compounds here (a specialty of Klapötke’s group), and the question is not whether such things are going to be explosive hazards. (That’s been settled by their empirical formulas, which generally look like typographical errors). The question is whether you’re going to be able to get a long enough look at the material before it realizes its dream of turning into an expanding cloud of hot nitrogen gas.
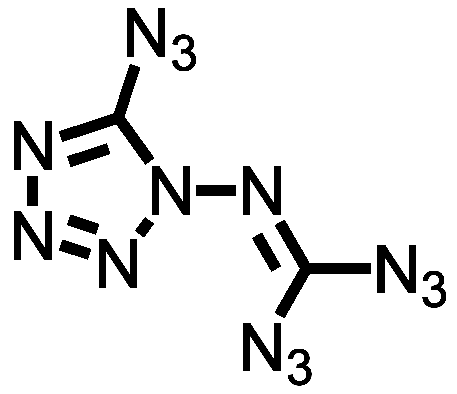
It’s time for another dispatch from the land of spiderweb-cracked blast shields and “Oh well, I never liked that fume hood, anyway”. Today we have a fine compound from this line of work, part of a series derived from N-amino azidotetrazole. The reasonable response to that statement is “Now hold it right there”, because most chemists will take one look at that name and start making get-it-away-from-me gestures. I’m one of them. To me, that structure is a flashing red warning sign on a dead-end road, but then, I suffer from a lack of vision in these matters.
But remember, N-amino azidotetrazole (I can’t even type that name without wincing) is the starting material for the work I’m talking about today. It’s a base camp, familiar territory, merely a jumping-off point in the quest for still more energetic compounds. The most alarming of them has two carbons, fourteen nitrogens, and no hydrogens at all, a formula that even Klapötke himself, who clearly has refined sensibilities when it comes to hellishly unstable chemicals, calls “exciting”. Trust me, you don’t want to be around when someone who works with azidotetrazoles comes across something “exciting”.
It’s a beast, all right. The compound is wildly, ridiculously endothermic, with a heat of formation of 357 kcal/mole, all of which energy is ready to come right back out at the first provocation (see below). To add to the fun, the X-ray crystal structure shows some rather strange bond distances, which indicate that there’s a lot of charge separation – the azides are somewhat positive, and the tetrazole ring somewhat negative, which is a further sign that the whole thing is trembling on the verge of not existing at all.
And if you are minded to make some yourself, then you are on the verge of not existing at all, either. Both the initial communication and the follow-up publication go out of their way to emphasize that the compound just cannot be handled:
Due to their behavior during the process of synthesis, it was obvious that the sensitivities (of these compounds) will be not less than extreme. . .The sensitivity of C2N14 is beyond our capabilities of measurement. The smallest possible loadings in shock and friction tests led to explosive decomposition. . .
Yep, below the detection limits of a lab that specializes in the nastiest, most energetic stuff they can think up. When you read through both papers, you find that the group was lucky to get whatever data they could – the X-ray crystal structure, for example, must have come as a huge relief, because it meant that they didn’t have to ever see a crystal again. The compound exploded in solution, it exploded on any attempts to touch or move the solid, and (most interestingly) it exploded when they were trying to get an infrared spectrum of it. The papers mention several detonations inside the Raman spectrometer as soon as the laser source was turned on, which must have helped the time pass more quickly. This shows a really commendable level of persistence, when you think about it – I don’t know about you, but one exploding spectrometer is generally enough to make recognize a motion to adjourn for the day. But these folks are a different breed. They ended up having to use a much weaker light source, and consequently got a rather ugly Raman spectrum even after a lot of scanning, but if you think you can get better data, then step right up.
No, only tiny amounts of this stuff have ever been made, or ever will be. If this is its last appearance in the chemical literature, I won’t be surprised. There are no conceivable uses for it – well, other than blowing up Raman spectrometers, which is a small market – and the number of research groups who would even contemplate a resynthesis can probably be counted on one well-armored hand.